 Reglan (Metoclopramide): Uses, Dosage, Side Effects, Interactions, Warning
Reglan (Metoclopramide): Uses, Dosage, Side Effects, Interactions, WarningCopyright © 2018 by RxList Inc. RxList does not provide medical advice, diagnosis or treatment. Reglan medicine (meoclopramide) Find the lowest prices in Reglan What is Reglan and how is it used? Reglan is a prescription drug used to treat symptoms of gastroesophageal reflux (GERD), nausea and vomiting caused by chemotherapy, diabetic gastroparesis and during the upper gastrointestinal tract. Rulen can be used alone or with other medicines. Rulen belongs to a class of drugs called antiemetic agents; prokinetic agents. What are the possible side effects of Reglan? Reglan may cause serious side effects, including: Get medical help right away, if you have any of the symptoms mentioned above. The most common side effects of Reglan include: Tell your doctor if you have any side effects that bother you or don't leave. These are not all possible side effects of Reglan. For more information, ask your doctor or pharmacist. Call your doctor to advise you about side effects. You may report side effects to FDA at 1-800-FDA-1088. WARNINGWARNINGTARDIVE DYSKINESIATARDIVE DYSKINESIATreatment with metoclopramide can cause tardive dyskinesia, a serious movement disorder that is often irreversible. The risk of developing late dyskinesia increases with the duration of treatment and the total cumulative dose. Treatment with metoclopramide can cause late dyskinesia, a severe movement disorder that is often irreversible. The risk of developing late dyskinesia increases with the duration of treatment and the total cumulative dose. Methoclopramide therapy should be suspended in patients who develop signs or symptoms of late dyskinesia. There is no known treatment for late dyskinesia. In some patients, symptoms may decrease or resolve after treatment of metoclopramide is stopped. Methoclopramide therapy should be suspended in patients who develop signs or symptoms of late dyskinesia. There is no known treatment for late dyskinesia. In some patients, symptoms may decrease or resolve after treatment of metoclopramide is stopped. Treatment with metoclopramide for more than 12 weeks should be avoided in all cases, but rare, where the therapeutic benefit is thought to exceed the risk of developing late dyskinesia. Treatment with metoclopramide for more than 12 weeks should be avoided in all cases, but rare, where the therapeutic benefit is thought to exceed the risk of developing late dyskinesia. See See See See DESCRIPTION For oral administration, rulen® tablets ( metoclopramide tablets, USP) 10 mg are white, scored, tablets in the form of "REGLAN" capsule on one side and "ANI 10" on the opposite side. Each tablet contains: Methopropramide Base 10 mg (such as monohydrochloride) Inactive Ingredients Magnesium Steate, Mannitol, Microcrystalline Cellulose, Acid.reglan® Stearic tablets ( metoclopramide tablets, USP) 5 mg are green tablets, in elliptical form recorded "REGLAN" on "5" on one side. Each tablet contains: Metoclopramide base 5 mg (such as monohydrochloride) Inactive Ingredients Maize Starch, D Yellow Cells 10 Aluminium Lake, FD Blue Pulido 1 Aluminium Lake, Lactose, Microcrystaline Cellulose, Silicone Dioxide, Steronic Acid. Methoclopramide chloride is a white crystalline substance, without odor, freely soluble in water. Chemistry is monohydrate of 4 amino-5-chloro-N- monohydrate[2-(diethylalmino)ethyl]-2-metoxi benzamide monohydrochloride. Its molecular formula is C14H22ClN3O2 • HCl • H2O. Its molecular weight is 354.3. INDICATIONS It is recommended to use den® tablets only for adults. Therapy should not exceed 12 weeks in duration. It is recommended to use den® tablets only for adults. Therapy should not exceed 12 weeks in duration. Symptom Gastroesofage Refluxreglan® tablets are indicated as short-term therapy (4 to 12 weeks) for adults with symptomatics, documented that it does not respond to conventional therapy. The main effect of metoclopramide is on symptoms and the day with less effect observed in night symptoms. If symptoms are limited to particular situations, such as after night meal, use of metoclopramide as unique doses before the provocative situation should be considered, instead of using the medication all day. Healing of ulcers and erosion has been shown endoscopically at the end of a 12-week trial using 15 mg q.i.d. As there is no documented correlation between symptoms and healing of esophageal lesions, patients with documented injuries should be monitored endoscopically. Diabetic Gastroparesis (Diabetic Gastrics) Diabetic Gastroparesis (Diabetic Gastrics) tablets ( metoclopramide tablets, USP) is indicated for the relief of symptoms associated with the Acute and diabetic. The usual manifestations of delayed gastric emptiness (e.g., nausea, acidity, persistence of fullness after meals, and ) seem to respond to rulen® within different time intervals. Significant relief of nausea occurs early and continues to improve on three-week period. vomiting and anorexia relief may precede the relief of the abdominal fullness by one Week or more. SLIDESHOWDOSAGE AND ADMINISTRATIONTherapy with the tablet rule should not exceed 12 weeks of duration. Rule tablet therapy should not exceed 12 weeks. For the relief of symptomatic gastroesophageal refluxAdminister from 10 mg to 15 mg of metoclopramide richloride, USP) orally up to 30 minutes before each meal and at bedtime, depending on the treated symptoms and clinical response (see and INDICATIONS AND USAGE). If symptoms occur only intermittently or at specific times of the day, the use of metoclopramide in individual doses up to 20 mg before the provocation situation may be preferred instead of continuous treatment. From time to time, patients (like as older patients) who are more sensitive to the therapeutic or adverse effects of metoclopramide It requires only 5 mg per dose. CLINESE PHARMACOLOGY AND ESOGEEL experience with esophageal erodes and ulcers is limited, but healing has so far been documented in a controlled trial using q.i.d. therapy to 15 mg/ dose, and this regime should be used when the injuries are present, as long as it is tolerated (see ). Because of the poor correlation between symptoms and endoscopic appearance of , targeted therapy Esophageal lesions are best guided by endoscopic evaluation. REACTIONS Therapy has not been evaluated for more than 12 weeks and cannot be recommended. For the relief of symptoms As it is based on diabetic gas tubes is (Diabetic Gas tric Stas is ) Manage 10 mg metoclopramide 30 minutes before each meal and at bedtime for two to eight weeks, depending on the response and the probability of continued well-being in drug discontinued. The initial path of administration should be determined by the severity of the symptoms presented. Yeah. only the first manifestations of diabetic gastric stasis are present, the oral rulen® administration can initiated. However, if there are serious symptoms, therapy should start with metoclopramide injection (check injection labeling before starting administration). Managing metoclopramide injection up to 10 days may be necessary before symptoms dissolve, in what time can be instituted oral administration. Since diabetic gastric stasis is frequently recurrent, Rulen® therapy should be reinstated to the first demonstration. Use in patients with kidney or liver deficiencySince metoclopramide is excreted mainly through the kidneys, in patients whose creatinine is the cleaning is less than 40 mL/min, the therapy should start approximately half of the recommended dose. Depending on clinical considerations of efficacy and safety, the dose can be increased or decreased as appropriate. See section for information about . OVERDOSEMetoclopramide suffers minimal liver except for simple conjugation. Your safe use has is described in patients with advanced kidney function that was normal. Each white tablet, in the form of a capsule, with rulen® stitching ( metoclopramide tablet, USP) contains 10 mg metoclopramide base (such as monohydrochloride). Available in: 100 tablet bottles (NDC 62559-166-01)NDC Each green tablet, in elliptical form ( metoclopramide tablets, USP) contains 5 mg metoclopramide base (such as monohydrochloride). Available in:Bottles of 100 tablets (NDC 62559-165-01)NDC Dispensing Tables In Tight, Container Resistant to Light. Tables should be stored at controlled ambient temperature, between 20°C and 25°C (68°F and 77°F). Manufactured by: ANI Pharmaceuticals, Inc. Baudette, MN 56623. Reviewed: Dec 2014SIDE EFFECTSIn general, the incidence of adverse reactions correlates with the dose and duration of metoclopramida administration. The following reactions have been reported, although in most cases the data is not allow a frequency estimate: CNS EffectsIdeality, drowsiness, fatigue, and occurs in approximately 10% of patients who receive more commonly prescribed dose of 10 mg q.i.d. (see ). Insomnia, headache, , dizziness or mental depression with suicidal ideation (see ) occurs less Often. The incidence of drowsiness is higher in higher doses. There are isolated reports seizures without clear relation to metoclopramide. Rarely, they've been PRECAUTIONSWARNINGSExtrapyramidal Reactions (EPS)Acute dystonic reactions, the most common type associated with metoclopramide, occurs in approximately 0.2 per cent of patients (1 in 500) treated with 30 to 40 mg metoclopramide per day. Symptoms include extremities, facial furniture, , eye crisis, rhythmic protrusion of the tongue, type of speech, , opisthotonus (-like reactions), and, seldom, stridor and possibly due to laryngospasm; usually these symptoms are easily reverted by (see ). Symptoms similar to parkinsonians may include , , wheel stiffness, similar to the mask (see ). ATTENTIONS are more frequently characterized by involuntary movements of the tongue, face, mouth, or jaw, and sometimes by involuntary movements of the trunk and/or extremities; movements can be coralofetotic in appearance (see ). WARNINGSMotor restlessness () may consist of feelings of anxiety, agitation, jitteriness, and insomnia, as as well as the inability to sit still, pacify, play on foot. These symptoms may disappear spontaneously or respond to a dose reduction. Neuroleptic Malignant Syndrome Cases of Syndrome (NMS) have been reported. This potentially fatal syndrome is composed of the complex symptom of , altered consciousness, autonomic stiffness and dysfunction (see ). WARNINGSDisturbing dedocrine, , secondary to hyperprolactinemia (see ). Retention of fluids secondary to transient elevation of (see ). PRECAUTIONSCLINICAL PHARMACOLOGYCardiovascular, , , fluid retention, acute congestive and possible AV block (see and ).CONTRAINDICATIONSPRECAUTIONSGastrointestinalNausea and intestinal disorders, mainly diarrhea. HepaticRarly, cases of hepatotoxicity, characterized by findings such as and altered liver function tests, when metoclopramide was administered with other drugs with known potential. Kidney Frequency and .Hematologic A few cases of , , or , generally without clear relationship with metoclopramida. , in adults and especially with overdose in neonates (see ). Adult sulfhemoglobinemia. OVERDOSE Allergic reactions Some cases of rash, , or bronchospasm, especially in patients with a history of . Rarely, edema angioneurotica, including or edema. Several visual disorders. . RUG INTERACTIONS The effects of metoclopramide on motility are antagonized by drugs and analgesics. Additive effects can occur when metoclopramide is given with alcohol, sedatives, hypnotics, narcotics or tranquilizers. The finding that metoclopramide releases catecholamines in patients with hypertension suggests that it should be used cautious, if at all, in patients who receive oxidase monoamine inhibitors. Absorption of stomach medications may be decreased (e.g. digoxin) by metoclopramide, while the rate and/or the extent of the absorption of the medications of the drug can be increased (e.g. , , levodopa, ethanol, cyclosporine). ( ) may be responsible for poor diabetic control in some patients. The exogenous administration can begin to act before the food has left the stomach and lead to . Because the action of metoclopramide will influence the delivery of food to the intestines and therefore the absorption rate, insulin dose, or dose time may require adjustment. WARNINGS Mental depression has occurred in patients with and without previous depression. Symptoms have varied from mild to severe and included suicidal ideation and suicide. The metoclopramide must be given to patients with prior depression history only if the expected benefits exceed the potential risks. Extrapyramidal symptoms, manifested primarily as acute dystonic reactions, occur in approximately 1 in 500 patients treated with the usual doses of adults from 30 to 40 mg/day of metoclopramide. These generally are seen during the first 24 to 48 hours of treatment with metoclopramide, occur more often in Pediatric patients and adult patients under 30 years of age and are even more frequent in higher doses. These symptoms may include movements of facial limbs and muscles, , ocular crisis, rhythmic protrusion of the tongue, type of speech, or dystonic reactions . Rarely, dystonic reactions can present as a stridor and possibly due to laryngospasm. If these symptoms should occur, inject 50 mg of diphenhydrate chloride intramuscularly, and they will usually collapse. Mesylate of benztropin, 1 to 2 mg intramuscularly, can is also used to reverse these reactions. Symptoms similar to Parkinsonian have occurred, most commonly within the first 6 months after beginning treatment with metoclopramide, but occasionally after longer periods. These symptoms usually replace within 2 to 3 months after the interruption of metoclopramide. Pre-existence patients Parkinson's disease should be administered cautiously, if not at all, as these patients may experience exacerbation of parkinsonian symptoms by taking metoclopramide. Dyskinesia Tardiva(See )(See )Treatment with metoclopramide can cause (), a potentially irreversible and Disfigurement disorder characterized by involuntary movements of the face, tongue or extremities. The risk of late increases with the duration of treatment and the accumulated total dose. An analysis of usage patterns showed that about 20% of patients using metoclopramide He took it for over 12 weeks. Treatment with metoclopramide for longer than recommended 12 weeks should be avoided in all cases, but rare, where the therapeutic benefit is thought to exceed the risk development of TD. Although the risk of developing TD in the general population can be increased among older persons, women and diabetics, it is not possible to predict which patients will develop metoclopramide TD. Both the risk of developing TD and the likelihood that TD becomes an irreversible increase duration of treatment and total cumulative dose. metoclopramide should be suspended in patients who have signs or symptoms of DTT. There's no known effective treatment for established cases of DTT, although in some patients DTT may refer, partial or completely, within several weeks to months after metoclopramide is withdrawn. The metoclopramide itself may delete, or partially delete, the signs of TD, thus masking the underlying disease process. The effect of this symptomatic deletion in the long-term course TD is unknown. Therefore, metoclopramide should not be used for the symptomatic control of TD. Neuroleptic Malignant Syndrome (NMS)There have been rare reports of an uncommon but life-threatening symptom complex at times referred to as Syndrome (NMS) associated with metoclopramide. Clinic manifestations of NMS include , muscle stiffness, altered consciousness and evidence of autonomic instability (irregular or arterial pressure, diphoresis and heart rate arrhythmias). Diagnostic evaluation of patients with this syndrome is complicated. When you arrive at a diagnosis, it is important to identify cases where clinical presentation includes both a serious medical disease (e.g., , systemic infection, etc.) and extrapyramid signs not treated or treated inadequately symptoms (). Other important considerations in the toxicity, , drug fever and primary central nerve system (CNS) . NMS management should include 1) immediate interruption of metoclopramida and others medicines not for concurrent therapy, 2) intensive and medical monitoring, and 3) treatment of any serious medical problem that is concomitant for which specific treatments are available. Bromocriptine and dantroleno sodium have been used in treating NMS, but their effectiveness has has not been established (see ). ADVERSE REACTIONSPRECAUTIONSGeneral In a patient study, metoclopramide was shown in the IV. catecholamines; therefore, caution should be exercised when metoclopramide is used in patients with . Because metoclopramide produces a transient increase in plasma, certain patients, especially those with or , may be at risk of developing fluid retention and volume overload. If these side effects occur at any time during metoclopramide therapy, the medication It must be suspended. Adverse reactions, especially those involving the nervous system, can occur after stopping the use of rulen®. A small number of patients may experience a withdrawal period after stopping the rule® that could include dizziness, nervousness and/or headaches. Patient Information Den® use is recommended only for adults. Methoclopramide may harm mental and/or physical skills required for the performance of dangerous tasks such as operating machinery or driving a motor. The outpatient should be warned accordingly. For additional information, patients should be instructed to view the Medicaments Guide to Rulen® tablets. Carcinogenesis, Mutagenesis, fertility deficiencyA 77-week study was performed in oral-dose rats up to about 40 times the recommended maximum daily human dose. Metoclopramide elevates levels and elevation persists during chronic administration. Weapon culture experiments indicate that approximately one third of human breast cancers are in vitro dependent on prolactin, a potential factor if the prescription of metoclopramide is contemplated in a patient previously detected. Though disturbing as , , , and have been reported with prolactin-elevating medicines, the clinical meaning of high levels of serum prolactin is unknown to most patients. An increase of breast neoplasms has been found in rodents after the chronic administration of prolactinstimulation Neuroleptic drugs and metoclopramide. No clinical studies or epidemiological studies carried out to date, however, have demonstrated an association between the chronic administration of these drugs and breast tumorigenesis; the available evidence is too limited to be conclusive at this time. An Ames mutagenic test performed in metoclopramide was negative. Pregnancy Category BReproduction studies made in rats, mice and rabbits by the I.V., I.M., S.C. and oral routes maximum levels ranging from 12 to 250 times the human dose has not shown deterioration fertility or significant damage to the fetus due to metoclopramide. There is, however, enough and well-controlled studies in pregnant women. Because animal reproduction studies not always Predictive of the human response, this medication should be used during pregnancy only if it is clearly necessary. Mothers of NursingMetoclopramide is excreted in human milk. Caution should be exercised when metoclopramide is administered to a nursing mother. Paediatric UseThe safety and efficacy in pediatric patients have not been established (see ). OVERDOSECare must be exercised in the administration of metoclopramide to neonates as prolonged authorization can produce excessive serum concentrations (see - Pharmacokinetics). In addition, the neonates have reduced the levels of NADH-cytochrome b5 reductase that, in combination with the mentioned pharmacokinetic factors, make the newborns more susceptible (see ) PHARMACOLOGY-CLANIC Pharmacokinetics The safety profile of metoclopramide in adults cannot be extrapolated to pediatric patients. Dystonias and other extrapyramidal reactions associated with metoclopramide are more common in pediatrics population than in adults. (See the warnings and - Extrapyramidal Reactions.) WARNING REACTIONS Extrapyramidal Reactions. Geriatric UseClinical studies rulen® did not include enough numbers of subjects over 65 years of age to determine if older people respond differently to younger subjects. The risk of developing side effects similar to Parkinsonians increases with the upward dose. Gériat patients you should receive the lowest dose of rulen® that is effective. If parkinsonian type symptoms develop in a geriatric patient who receives rule®, should generally be suspended before starting any rule Specific antiparkinsonian agents (see WARNINGS and – For the relief of the symptomatic gas trousers ophageal reflux). WARNINGSDOSAGE AND ADMINISTRATION - For the relief of symptomatic gas trots ophageal reflux The elderly may be at greater risk of late dyskinesia (see WARNINGS – Tardive Dys kinesia). WARNINGS– Tardive Dys kinesiaSedation has been reported to rulen® users. The building can cause and manifest itself as oversed in the elderly (see , PRECAUTIONS – Information for Patients and – Effects of CNS). CLINICA PHARMACOLOGY – Information for DURSO REACTIONS – SNC Efectsreglan® is known for being substantially excreted by the kidney, and the risk of toxic reactions to this medication may be greater in patients with deficient kidney function (see – Use in patients with kidney or liver disabilities. DOSAGE AND ADMINISTRATION– Use in patients with kidney or liver disabilities For these reasons, the selection of doses for an older patient should be cautious, usually starting with the low end of the dosage range, reflecting the increased frequency of renal function decrease, concomitant disease or other drug therapy in the elderly (see – For Symptom Gastroesophageal Reflux Relief and Use in Patients with Renal or Hepatic - The deterioration. DOSAGE AND ADMINISTRATION– For the Symptom Gastroesophageal Reflux Relief and Use in Patients with Renal or Hepatic Less Other Special Populations Patients with NADH-citocromo Reductase b5 deficiency is at greater risk of developing methemoglobinemia and/or sulfhemoglobinemia when metoclopramide is administered. In patients experience methemoglobinemia induced metoclopramide, methylene blue treatment is not recommended (see ). OVERDOSEOVERDOSSymptoms of overdose may include drowsiness, disorientation, and extrapyramid reactions. or antiparkinson medications or with anticholinergic properties may be useful to control extrapyramidal reactions. Symptoms are self-limiting and usually disappear within 24 hours. eliminates relatively little metoclopramide, probably due to the small amount of the blood drugs relative to tissues. Similarly, the continuous outpatient does not eliminate Significant amounts of drugs. It is unlikely that the dose needs to be adjusted to compensate loss through . Dialysis is not likely to be an effective method of drug elimination in overdose situations. Unintentional overdose has been reported due to mismanagement in infants and children with the use of children metoclopramide oral solution. While there was no standard pattern for reports associated with those reports overdose, events including seizures, extrapyramidal reactions, and . has occurred in premature and long-term neonates that received overdose of metoclopramide (1 to 4 mg/kg/day, intramuscular or intravenous for 1 to 3 or more days). Methylene blue intravenous administration can reverse methemoglobinemia. However, Methylene blue can cause in patients with , which can be fatal (see – Other Special Populations). PRECAUTIONS Special populationsSLIDESHOWCONTRAINDICATIONSMetoclopramide should not be used every stimulation of motility might be dangerous, for example, in the presence of gastrointestinal, mechanical obstruction or perforation. Methoclopramide is contraindicated in patients with because the medicine can cause a probably due to the release of catecholamines from the tumor. Tal. crises can be controlled by phenolamine. metoclopramide is contraindicated in patients with known sensitivity or intolerance to the medication. metoclopramide should not be used in epileptics or patients who receive other drugs that are likely to be cause extrapyramidal reactions, as the frequency and severity of seizures or extrapyramidal reactions can be increased. PHARMACOLOGY CLÍNICAMetoclopramide stimulates the motility of the upper part without stimulating , Or pancreatic secretions. Your mode of action is not clear. It seems to sensitize tissues to action the effect of metoclopramide on motility does not depend on intact vagal, But it can be abolished by drugs. metoclopramide increases the tone and breadth of gastric contractions (especially antral ones) piloric sphincter and bulb, and increments of and result in gastric emptied and intestinal transit. Increase the tone of the lower part Sphincter. It has little effect, if any, on the motility of the or . In patients with and low LESP (lower esophageal sphincter), only oral doses of metoclopramide produce dose-related increases in LESP. Effects start at about 5 mg and increase to 20 mg (the most tested dose). The increase in LESP of a dose of 5 mg lasts about 45 minutes and 20 mg lasts between 2 and 3 hours. Increase in the rate of stomach emptied has been observed with individual oral doses of 10 mg. The antiemetic properties of metoclopramide appear to be the result of its central antagonism and Peripheral receivers. Dopamine produces nausea and stimulation of the spinal cord chemoreceptor zone (CTZ), and metoclopramide blocks the stimulation of CTZ by agents such as ldopa or apomorphine that is known to increase dopamine levels or possess dopamine-like Effects. Methoclopramide also abolishes the deceleration of gastric emptied caused by apomorphine. Like phenothiazins and related drugs, which are also dopamine antagonists, metoclopramida produces sedation and can produce extrapyramid reactions, although they are comparatively rare (see ) Methoclopramide inhibits the central and peripheral effects of apomorphine, induces liberation and cause a transient increase in circulating levels, which can be associated with transient fluid retention. WARNING The beginning of the pharmacological action of metoclopramide is 1 to 3 minutes after an intravenous dosage, 10 to 15 minutes after administration, and 30 to 60 minutes after an oral doses; pharmacological effects persist for 1 to 2 hours. PharmacokineticsMetoclopramide is fast and well absorbed. Concerning an intravenous dose of 20 mg, the absolute oral bioavailability of metoclopramide is 80% ± 15.5% as shown in 18 subject. The peak plasma concentrations occur at about 1 to 2 hr after a single oral dose. Time similar to The peak is observed after individual doses in a stable state. In a single dose study of 12 subjects, the area under the drug concentration curve increases linearly with doses of 20 to 100 mg. Peak concentrations increase linearly with doses; peak time concentrations remain the same; total cleaning of the body does not change; and the elimination rate remains Same. The average life of elimination in individuals with normal kidney function is 5 to 6 hr. Linear processes properly describe the absorption and removal of metoclopramide. Approximately 85% of the radioactivity of an orally administered dose appears in the urine within 72 hr. Of 85% eliminated in the urine, approximately half is present as free or conjugated metoclopramide. The drug is not widely linked to plasma proteins (about 30%). All volume of the body distribution is high (approximately 3.5 L/kg) which suggests a wide distribution of drugs to tissues. Kidney deterioration affects the cleaning of metoclopramide. In a study with patients with varying degrees renal impairment, a reduction in creatinine cleaning was correlated with a plasma reduction cleansing, renal demining, non-dryntal demining and increased elimination of the average life. The one However, metoclopramide in the presence of renal deficiency remained linear. Reduction in cleansing as a result of kidney impairment suggests that the downward adjustment of the maintenance dose must be done to avoid the accumulation of drugs. Adult Pharmacokinetics Data Adult Pharmacokinetics Data Parameter Value Vd (L/kg) - 3.5. Plasma Protein Binding ~ 30% t1/2(hr) 5-6 Oral bioavailability 80%±15.5% In pediatric patients, pharmacodynamics of metoclopramide after oral and intravenous administration is very variable and a ratio of concentration effect has not been established. There is not enough reliable data to conclude if metoclopramide pharmacokinetics in Adults and the pediatric population are similar. Although there is insufficient data to support metoclopramide effectiveness in symptomatic pediatric patients (GER) or cancer -related, its pharmacokinetics have been studied in these patient populations. In an open label study, six pediatric patients (age range, 3.5 weeks to 5.4 months) with GER received metoclopramide 0.15 mg/kg oral solution every 6 hours for 10 doses. The average peak plasma metoclopramide concentration after the tenth dose was 2 times (56.8 μg/L) higher than that observed after the first dose (29 μg/L) indicating the accumulation of drugs with repeated doses. After the tenth dose, average time to reach peak concentrations (2.2 hr), average life (4.1 hr), cleaning (0.67 L/h/kg), and distribution volume (4.4 L/kg) of metoclopramide were similar to those observed after the first dose. In the younger patient (age, 3.5 weeks), the average life of metoclopramide after the first and tenth dose (23.1 and 10.3 hr, respectively) were significantly longer compared to other infants due to reduction authorization. This can be attributed to immature liver and kidney systems at birth. Unique intravenous doses of metoclopramide 0.22 to 0.46 mg/kg (average, 0.35 mg/kg) more than 5 minutes to 9 patients with pediatric cancer who receive chemotherapy (average age, 11.7 years; range, 7 to 14 yr) for induced vomiting. Methoclopramide plasma concentrations extrapolated at zero time range from 65 to 395 μg/L (average, 152 μg/L). Half-life elimination, the cleaning and distribution volume of metoclopramide were 4.4 hr (range, 1.7 to 8.3 hr), 0.56 L/h/kg (range, 0.12 to 1.20 L/h/kg), and 3.0 L/kg (range, 1.0 to 4.8 L/kg), respectively. In another study, nine patients with pediatric cancer (age range, 1-9 yr) received from 4 to 5 intravenous patients infusions (more than 30 minutes) of metoclopramide at a dose of 2 mg/kg to control. After the last dose, the maximum serum concentrations of metoclopramide ranged from 1060 to 5680 μg/L. Average half of life, cleaning and distribution volume of metoclopramide were 4.5 hr (range, 2.0 to 12.5 hr), 0.37 L/h/kg (range, 0.10 to 1.24 L/h/kg), and 1.93 L/kg (range, 0.95 to 5.50 L/kg), respectively. PATIENT INFORMATIONREGLAN (REG-lan) TabletsREGLAN (REG-lan) Read the Medication Guide that comes with REGLAN before you start taking it and every time you get a Fill in. There may be new information. If you take another product containing metoclopramide (like REGLAN injection, ODT REGLAN or metoclopramide oral solution, read the Medication Guide that comes with that product. Some of the information can be different. This medicine guide do not take the place to talk to your doctor about your medical condition or your treatment. What is the most important information you should know about REGLAN? What is the most important information you should know about REGLAN? REGLAN can cause serious side effects, including: Sensitive dyskinesia (abnormal muscle movements). These movements occur mainly in the face muscles. You can't control these movements. They may not leave even after stopping REGLAN. There is no treatment for , but symptoms may decrease or disappear over time after you Stop taking REGLAN. Dskinesia Tardiva (abnormal muscle movements). Your chances of getting late go up: Your doctor may not know if you will receive late dyskinesia if you take REGLAN. Call your doctor right away if you get movements that you can't stop or control, such as:See the section "What are the possible side effects of REGLAN?" for more information on the side Effects. What's REGLAN? What's REGLAN? REGLAN is a prescription drug used: It is not known if REGLAN is safe and works in children. Who shouldn't take REGLAN? Who shouldn't take REGLAN? Do not take REGLAN if you: What should I tell my doctor before taking REGLAN? What should I tell my doctor before I take REGLAN? Tell your doctor about all your medical conditions, even if you have: Tell your doctor about all your medical conditions,tell your doctor about all medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements. REGLAN and some other medicines can interact with one to the other and may not work too, or cause possible side effects. Do not start any new medication while taking REGLAN until you talk to your doctor. Tell your doctor about all the medications you take, including prescription and prescription medicines, vitamins and herbal supplements. Especially tell your doctor if you take: Especially tell your doctor if you take: If you are not sure if your medication is listed above, ask your doctor or pharmacist. He knows the medications you take. Keep a list of them and show it to your doctor and pharmacist when you Get a new medication. How should I take REGLAN? How should I take REGLAN? What should I avoid while taking REGLAN? What should I avoid while taking REGLAN? What are the possible side effects of REGLAN? What are the possible side effects of REGLAN? Reglan may cause serious side effects, including: Reglan may cause serious side effects, including: Sensitive dyskinesia (abnormal muscle movements). Uncontrolled spasms of the muscles of the face and neck, or muscles of your body, arms and legs (distony). Depression, thoughts about suicide and suicide. Neuroleptic malignant syndrome (NMS). Parkinsonism. Call your doctor and get medical help right away if: Call your doctor and get medical help right away if: Common Rulen side effects include: Common Rulen side effects include: You may have more side effects the longer you take REGLAN and more REGLAN take. It may still have side effects after stopping REGLAN. You may have symptoms of stopping REGLAN (retired) as headaches, and feeling dizzy or nervous. Tell your doctor about any side effects that bother you or don't leave. These aren't all possible side effects of REGLAN. Call your doctor to advise you about side effects. You may report side effects to FDA at 1-800- FDA-1088. How can I store REGLAN? How can I store REGLAN? Keep REGLAN and all medicines out of reach of children. Keep REGLAN and all medicines out of reach of children. General information about REGLANGeneral information about REGLANMedicines are sometimes prescribed for purposes other than those listed in a Medication Guide. No. use REGLAN for a condition for which it was not prescribed. Don't give other people a ERW, even if they have the same symptoms you have. It can damage them. This Drug Guide summarizes the most important information about REGLAN. If you want more information, talk to your doctor. You can ask your doctor or pharmacist for information about REGLAN is written for health professionals. For more information, go to www.anipharmaceuticals.com or call-free at 1-800-308-6755. What are the ingredients in REGLAN? What are the ingredients in REGLAN? Active ingredient: metoclopramideActive ingredients:Inactive ingredients :REGLAN 10 mg tablets : magnesium stearate, manitol, microcrystalline cellulose, 10 mg estearic acidREGLAN :REGLAN 5 mg tablets : corn starch, D yellow plague 10 aluminum lake, FD Blue lake 1 lake lactose, cellulose microcrystalline, silicon dioxide, estearic acidREGLAN 5 mg tablets : From Report Problems to the Food and Drug AdministrationYou are encouraged to report negative side effects of prescription drugs to the FDA. Visit the website or call 1-800-FDA-1088. Health Solutions of Our SponsorsQuick identification tool, easy, pill identification Drug Interaction Tool Drug Powerful Interaction Control Pharmacy Locater Tool Including 24 Hours, PharmaciesReglan Digital print print print print print print print, white, printed with 2203, TEVAround, white, printed with TV, 2204round, white, printed with WPI, 22 29xround, white, printed with R.21 RxList does not provide medical advice, diagnosis or treatment. .
Metoclopramide: 7 things you should knowMedically reviewed by . Last updated on November 9, 2020. Councils1. How it works2. The other way around. If you are between ages 18 and 60, do not take any other medication or do not have other medical conditions, the side effects you are most likely to experience include: Note: Generally, elderly or children, people with certain medical conditions (such as liver or kidney problems, heart disease, diabetes, seizures) or people who take other medicines are at greater risk of developing a wider range of side effects. Note:4. Metaclopramide of the fund can be used in the short term for the treatment of gastrointestinal reflux disease that does not respond to other medications or to treat diabetic stasis. Side effects may include sedation and movement disorders such as muscle stiffness and late dyskinesia.5. Tips6. Response and effectiveness7. InteractionsDoctors who interact with metoclopramide may decrease their effect, affect the time it works, increase side effects or have less effect when taken with metoclopramide. An interaction between two medications does not always mean that you should stop taking one of the medications; however, it sometimes does. Talk to your doctor about how drug interactions should be managed. Common medicines that can interact with metoclopramide include: Note that this list is not all inclusive and includes only common medicines that can interact with metoclopramide. You should refer to the prescription information for metoclopramide for a complete list of interactions. More about metoclopramide Consumer resourcesProfessional remediesRelated treatment guidelinesReferencesMetoclopramide. Revised: 05/2020. Drugs.com Learn moreRemember, keep this and all other medicines out of reach of children, never share your medications with others, and use metoclopramide only for the prescribed indication. Always consult your healthcare provider to ensure that the information shown on this page applies to your personal circumstances. Copyright 1996-2021 Drugs.com. Date reviewed: November 8, 2020. State of drugs Availability Only required Rx CSA Table* It's not a controlled drug. N/A Loading... History of approval History of drugs in the FDA Loading..., , , , , , , , , , 4.1Drugs.com Mobile Apps The easiest way to find information about drugs, identify pills, check interactions and set up your own personal drug records. Available for Android and iOS devices. SupportAboutTerms " Privacy to Drug.com newsletters for latest drug news, new drug approvals, alerts and updates. Drug.com provides accurate and independent information about more than 24,000 prescription drugs, free-sale medicines and natural products. This material is provided only for educational purposes and is not intended for medical advice, diagnosis or treatment. Data sources include IBM Watson Micromedex (updated 3 Mar 2021), Cerner MultumTM (updated 1 Mar 2021), ASHP (updated 3 Mar 2021) and others. Ad options
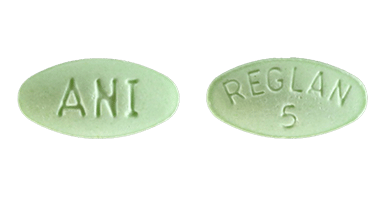
Reglan Side Effects | Common Reactions & Serious Risks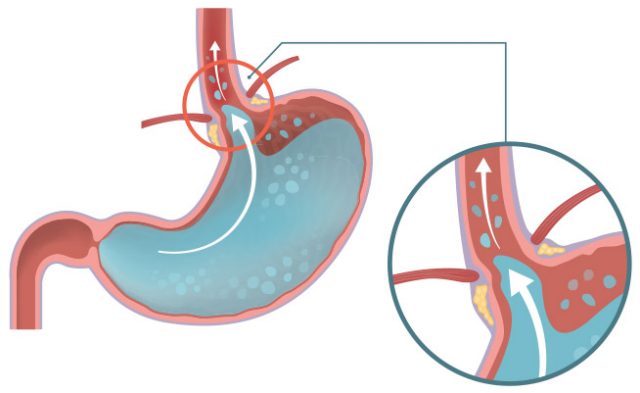
Reglan - FDA Black-Box Warning, Tardive Dyskinesia Risk
Metoclopramide (Reglan®)/how-long-does-morphine-stay-in-your-system-80288-FINAL-573f9ea42b4f4fbbac4fc5432b6d5c85.png)
How Long Does Morphine Stay in Your System?
Reglan (Metoclopramide (Oral/Injection)) - Side Effects, Interactions, Uses, Dosage, Warnings | Everyday Health
How long do drugs stay in your system? - Drug and Alcohol Information and Support in Ireland - Drugs.ie
Metoclopramide | Side Effects, Dosage, Uses, and More
Reglan - FDA Black-Box Warning, Tardive Dyskinesia Risk
Should you use Reglan for breastfeeding? | The Checkup
Reglan - FDA Black-Box Warning, Tardive Dyskinesia Risk
Metoclopramide (Reglan)
Reglan Injection (Metoclopramide Injection): Uses, Dosage, Side Effects, Interactions, Warning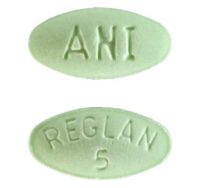
Reglan - FDA Black-Box Warning, Tardive Dyskinesia Risk
Reglan - FDA Black-Box Warning, Tardive Dyskinesia Risk
How Long Do Drugs Stay in Your System? - Addiction Center
Flexeril (Cyclobenzaprine) How Long Does It Stay In Your System? | The Recovery Village Drug and Alcohol Rehab
Metoclopramide Side Effects You Need To Know- Innovet Pet:max_bytes(150000):strip_icc()/what-is-the-24-hour-flu-770474_color1-5b95dbc34cedfd00256c4e66.png)
24-Hour Stomach Flu: Symptoms, Causes, and Treatment
How do you know if you have serotonin syndrome?
Sumatriptan: 7 things you should know - Drugs.com
Buy Generic Reglan In Australia Without Prescription - Australian Cheapest Drugs
The Safety of Metoclopramide Use in the First Trimester of Pregnancy | NEJM
Stalevo: Generic, dosage, side effects, alternatives, uses, and more
Metoclopramide Side Effects: Common, Severe, Long Term - Drugs.com
Reglan (Metoclopramide): Uses, Dosage, Side Effects, Interactions, Warning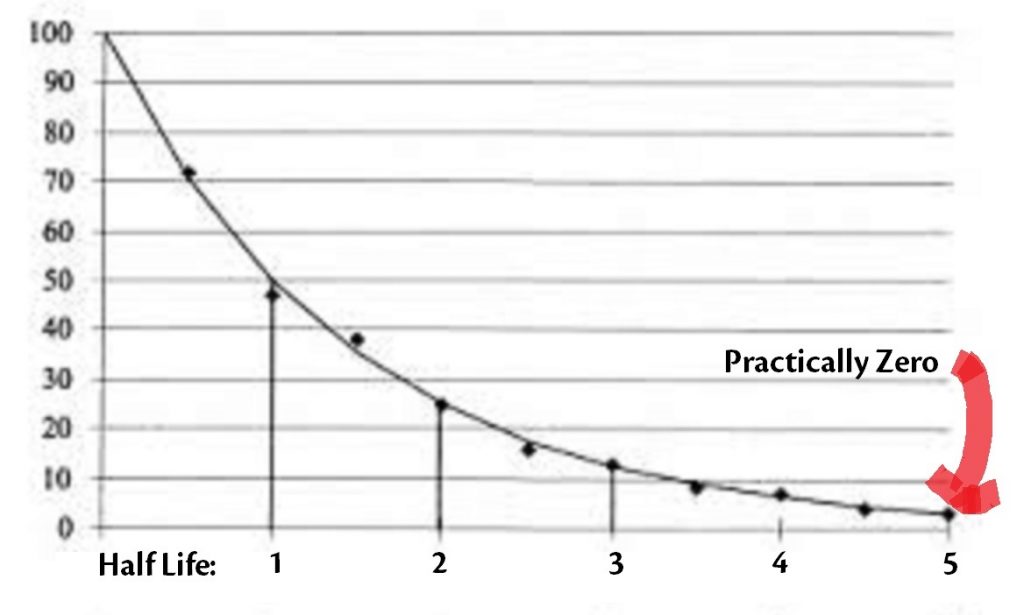
How Long Does It Take for a Medication To Go Away When I Stop Taking It? – Psych
Metoclopramide | Side Effects, Dosage, Uses, and More
Metoclopramide - Drugs and Lactation Database (LactMed) - NCBI Bookshelf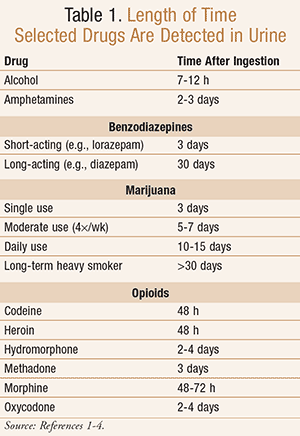
Urine Drug Screening: Minimizing False-Positives and False-Negatives to Optimize Patient Care/GettyImages-530695244-5c13cd5646e0fb000123e424.jpg)
How Long Does Marinol Stay in Your System?
Dimenhydrinate Withdrawal Symptoms & Detox | The Recovery Village
Morning Sickness: When It Starts, Symptoms, Remedies:max_bytes(150000):strip_icc()/young-woman-drug-addicted-social-problems-concept-sitting-thinking-of-taking-pills-1144911064-06cc8ccc0120407d9030307f713eeccb.jpg)
Medications You Should Never Mix With Alcohol
Metoclopramide as intermittent and continuous infusions in critically ill patients: a pilot randomized clinical trial | Journal of Comparative Effectiveness Research
Tardive Dyskinesia: Definition, Treatment, and Symptoms
The Safety of Metoclopramide Use in the First Trimester of Pregnancy | NEJM
Metoclopramide Injection - NPS MedicineWise
Antiemetic Drugs List: OTC, Side Effects, for Pregnancy, and More
Oral Cyclosporine Use in Dogs | Today's Veterinary Practice
 Reglan (Metoclopramide): Uses, Dosage, Side Effects, Interactions, Warning
Reglan (Metoclopramide): Uses, Dosage, Side Effects, Interactions, Warning

/how-long-does-morphine-stay-in-your-system-80288-FINAL-573f9ea42b4f4fbbac4fc5432b6d5c85.png)











:max_bytes(150000):strip_icc()/what-is-the-24-hour-flu-770474_color1-5b95dbc34cedfd00256c4e66.png)










/GettyImages-530695244-5c13cd5646e0fb000123e424.jpg)

:max_bytes(150000):strip_icc()/young-woman-drug-addicted-social-problems-concept-sitting-thinking-of-taking-pills-1144911064-06cc8ccc0120407d9030307f713eeccb.jpg)






Posting Komentar untuk "how long does reglan stay in your system"